Chemistry:Ortho-Diethynylbenzene dianion
| It has been suggested that meta-Diethynylbenzene dianion be merged into this page. (Discuss) Proposed since May 2018. |
| It has been suggested that para-Diethynylbenzene dianion be merged into this page. (Discuss) Proposed since May 2018. |
| |
| Names | |
|---|---|
| IUPAC name
Ortho-diethynylbenzene dianion
| |
| Properties | |
| C6H4C2−4 | |
| Related compounds | |
Related bases
|
Meta-diethynylbenzene dianion Para-diethynylbenzene dianion |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
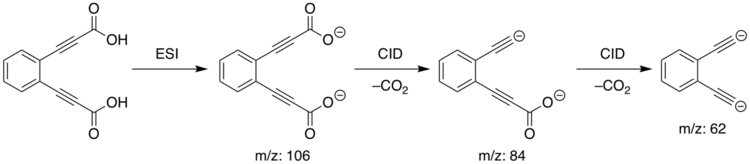
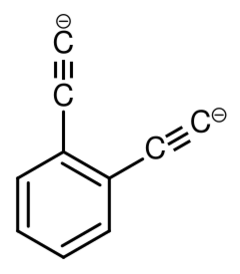
Ortho-diethynylbenzene dianion is a superbase with the chemical formula of [C6H4(C2)2]2−.[1][2] The base was created in mass spectroscopy experiments by researchers in Australia.[1]
Background
The largest possible proton affinity value for a base in a solution of water is that of hydroxide, 1,633.14 kJ/mol. Bases with higher proton affinities irreversibly abstract protons from water, and as a result the anions cannot be observed. Therefore, stronger bases must be used in an aprotic solvent such as tetrahydrofuran (oxolane). According to calculations, ortho-diethynylbenzene dianion is the strongest possible organic base, with a proton affinity of 1843 kJ mol−1.
Ortho-diethynylbenzene dianion has two isomers: Meta-diethynylbenzene dianion and Para-diethynylbenzene dianion. Because these ions would abstract protons from any organic solvent, they are known only in the gas phase.[1]
Synthesis and Observation
Since ortho-diethynylbenzene dianion cannot be made in solution, investigation of the dianion is difficult. In the gas phase, however, the absence of any solvent interaction provides an ideal environment for investigating the basicity of the compound, and the gas phase value of the proton affinity can be calculated with greater precision.
The synthesis of ortho-diethynlbenzene dianion was performed in a linear quadrupole mass spectometer. Electrospray Ionisation (ESI) of the diacid precursor results in the dicarboxylate dianion ([C6H4(C3HO2)2]) losing two Hydrogen ions to become ([C6H4(C3O2)2]2−). The dianion was mass-selected and then subjected to Collision Induced Dissociation (CID), resulting in the loss of two dioxide molecules and generation of the ortho-diethynylbenzene dianion:
The meta and para isomers were prepared analogously.
Reactions of the gas-phase dianions were studied by passing them, in a helium carrier gas, over a small amount of the reagent of interest. Reaction with heavy water produced a singly-deuterated monanion with m/z 126. Reaction with benzene produced the phenyl anion, with m/z of 77, highlighting the extreme basicity of the dianion. Attempted reaction with deuterium gas and deuterated methane was not successful despite the favourable thermodynamics; the authors attribute this to the high activation barrier for proton abstraction from those substrates.[1]
See also
- Lithium monoxide anion.
- Tetrahydrofuran. (Recently in 2013, the preferred IUPAC name has been changed to Oxolane)
- Lithium diisopropylamide.
- Sodium Hydride.
References
- ↑ 1.0 1.1 1.2 1.3 Poad, Berwyck L. J.; Reed, Nicholas D.; Hansen, Christopher S.; Trevitt, Adam J.; Blanksby, Stephen J.; Mackay, Emily G.; Sherburn, Michael S.; Chan, Bun et al. (2016). "Preparation of an ion with the highest calculated proton affinity: ortho-diethynylbenzene dianion". Chem. Sci. 7 (9): 6245–6250. doi:10.1039/C6SC01726F.

- ↑ Bergius, Will (19 July 2016). "Basically record breaking". https://www.chemistryworld.com/news/basically-record-breaking/1010062.article.